
Principal Investigator
Our team studies the molecular bases of protein quality control and age-associated diseases using C. elegans as multicellular model. Combining genetic and biochemical approaches, we pioneered organismal proteostasis mechanisms regulated by ubiquitin.
The maintenance of proteostasis in every cell type involves repair and degradation of defective proteins, which is essential for organismal physiology and longevity. The key interest of our laboratory is to understand how protein quality control systems are mechanistically regulated to overcome age-associated protein damage. Our recent work identified conserved disease mechanisms related to mitochondrial pathologies, diabetes, obesity, progressive myopathy, and neurodegenerative diseases.
We study protein degradation mechanisms with the ultimate goal of delaying the aging process and the aggregation of misfolded proteins for therapeutic interventions against degenerative disorders, such as Alzheimer’s, Huntington’s, and Parkinson’s disease.
Current research in the Hoppe lab particularly aims to understand the dynamic regulation of proteolytic pathways that integrate environmental and physiological changes. The detailed analysis of both cellular and tissue-related coordination of proteostasis will allow to assemble a global picture of conserved protein degradation networks important to safeguard the organismal proteome in health and disease. The Hoppe lab aims to unravel the interplay between proteolytic networks at the molecular, cellular, and organismal level and to define adaptation mechanisms that ensure its integrity in response to environmental stress conditions or inherited, disease-associated mutations. The central research program is focused on the following points:
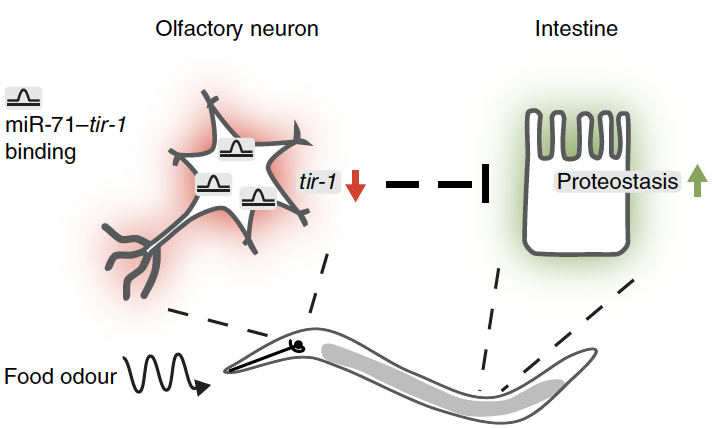
Thorsten Hoppe is one of the leading scientists in the field of protein homeostasis (proteostasis) from molecular mechanisms to physiology and disease. Using innovative genetic approaches in combination with biochemical technologies in the model organism C. elegans, he has pioneered the study of the ubiquitin/proteasome system from an organismal perspective. In his 25-year research career to date, he has regularly made outstanding contributions to the field. In his PhD thesis with Prof. Stefan Jentsch, he identified a new class of so-called E4 enzymes that regulate the extension of polyubiquitin chains on substrate proteins (Cell 1999) and identified unconventional regulation of membrane-bound proteins by the proteasome (Cell 2000, Cell 2001). Based on his in-depth knowledge of the ubiquitin system, he was the first to study protein degradation in C. elegans to better understand how proteostasis networks influence ageing (Cell 2004, Nat Cell Biol 2007, Cell Metab 2014). His group has also made numerous groundbreaking discoveries, including the identification of a muscle-specific quality control pathway fundamental to human myopathies (Cell 2004, Nat Cell Biol 2007, Cell 2013, Am J Hum Genet 2020) as well as chromatin-associated protein degradation mechanisms important for DNA replication and DNA damage response (PNAS 2008, Mol Cell 2011, Nat Comm 2016, Nat Struct Mol Biol 2016, Cell Rep 2021). He also contributed to one of the first publications on ubiquitin-dependent regulation of ageing (Nat Cell Biol 2011) and showed how ubiquitin-dependent degradation of the conserved insulin receptor affects the coordination between proteostasis and longevity (Cell 2017, Mol Cell 2022). In addition, his team characterized how neuronal perception of environmental stimuli such as odor or temperature changes influence physiological processes and ageing (Nat Metab 2019, Aging Cell 2022, Nat Comm 2022) and also identified a novel endoplasmic reticulum antiviral defense mechanism (Nat Cell Biol 2022). Prof. Thorsten Hoppe is committed to using interdisciplinary and innovation approaches to enable future novel therapeutic interventions for the treatment of age-related diseases in general and neurodegenerative disorders in particular.

Principal Investigator