
Principal Investigator
Director of Max Planck Institute for Metabolism Research
Director of Center for Endocrinology, Diabetes and Preventive Medicine
Our research focusses on elucidating the CNS-dependent regulation and age-dependent dysregulation of energy and glucose metabolism.
Our studies on CNS-dependent regulation of energy and glucose metabolism revealed a previously unappreciated role for insulin action in the CNS to control organismal glucose homeostasis and insulin sensitivity. We defined distinct Agouti-related peptide (AgRP)-expressing neurons in the hypothalamus as critical mediators of insulin's metabolic actions, revealed the molecular mechanisms of insulin action in these neurons as well as their alterations in obesity.
We aim to understand in detail how fundamental regulatory principles for food intake in the brain are linked to higher brain functions like emotions, or the smell and appearance of a particular food. The integration of all this information leads to the decision about when one eats – and what one eats – and as such whether one becomes overweight. These findings could have a decisive influence on developing new therapeutic approaches to obesity and diabetes mellitus.
We aim to define the mechanisms and consequences of age- and obesity-associated alterations of the newly identified regulation of hepatic proteostasis through the sensory food perception-dependent regulation of melanocortin neurons.
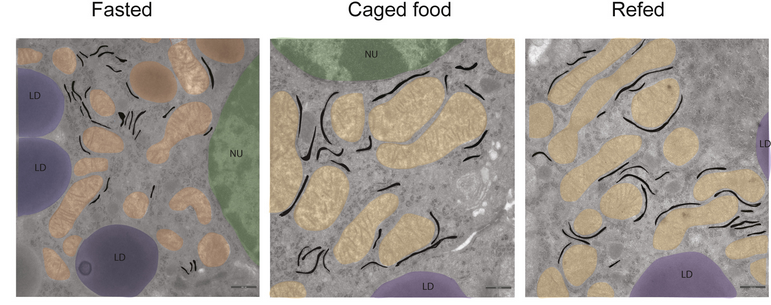
Contrary to the long-standing assumption that food intake and metabolism-regulating AgRP and POMC neurons are controlled by hormonal feedback signals that communicate the energy status of the organism – e.g., leptin, insulin - we have shown that sensory food perception already transiently regulates these neurons in exactly the same way as leptin and insulin do in the medium and long-term. We found that the sensory food perception-dependent activation of POMC neurons stimulates the sympathetic innervation of the liver to activate the non-cell autonomous activation of an ER stress response in this organ. This leads to a priming of liver proteostasis and thus to the preparation of the liver for postprandial activation of protein synthesis and secretion. Thus, highly specialized neurons which constantly monitor the energy state of the organism, as well as anticipated changes thereof, are critical regulators of physiological adaptations to changes in energy supply.

Principal Investigator
Director of Max Planck Institute for Metabolism Research
Director of Center for Endocrinology, Diabetes and Preventive Medicine