
Age-related decline in skin function is a major risk factor for skin fragility, impaired wound healing, and skin cancer but also perturbed whole-body physiology. We are investigating the molecular basis of age-related skin pathologies and how this knowledge can be exploited for the prevention and treatment of disease.
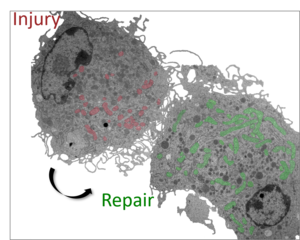
The skin provides a life-sustaining structural and immunological barrier of the organism. To ensure maintenance of proper structure and function, removal of damaged material and its replacement by regenerative processes needs to be tightly coordinated. Mechanisms driving this remarkable regenerative capacity throughout life are still only understood at a rudimentary level. Our group’s aim is to identify regulators and effectors by which the skin orchestrates balanced defense while promoting regenerative responses to maintain skin barrier integrity and function during life. We also compare physiological regeneration to neoplastic growth, to delineate age-related corrupted repair programs as a tumor-promoting principle.
Awareness of the morbidity caused by age-related skin frailty is still underrepresented in today’s medical practice. Clinicians and patients need to be made aware that healthy aging of the entire organism requires healthy skin.
The Eming lab is aiming at deeper understanding of how cells sense tissue damage and how these events translate into a regenerative response or disease. The ultimate goal is to reprogram the healing response, in order to readjust postnatal repair into regeneration and to develop novel strategies for pharmacological interventions to facilitate healing of injured, diseased or aging tissues. The close interaction between basic science and clinical expertise enables the group to translate new findings into therapeutic approaches.
There is substantial evidence for an inverse correlation between immune competence and the tissue’s capacity to regenerate or repair itself by forming a scar. Studies in mammals with limited regenerative capacity and findings in non-mammalian model organisms with a high regenerative capacity have contributed to this paradigm. In the past decade, the Eming lab has made seminal contributions to unraveling the mechanisms by which the immune system intervenes in the healing response or its failure.
Using a cross-species analytical approach of wound tissues in mice, zebrafish, Drosophila and humans, they identified novel inflammatory proteases and targets in the wound environment and provided evidence on how their interactions disrupt downstream cell functions essential for repair.
These studies have led to the discovery of new molecular control mechanisms in tissue regeneration. The findings have provided insight into how engineered growth factors in concert with biomaterials may offer a potent molecular approach for therapeutic angiogenesis. The group has also developed new genetically modified mouse models and techniques to investigate how monoytes/macrophages repair skin wounds and how these cells contribute to skin carcinogenesis. In collaboration with other investigators, they have recently identified new pathways that may underlie scarless repair in zebrafish but determine scar formation in mice and humans. The group has contributed to numerous clinical trials to optimize therapeutic strategies for conditions with impaired healing.
