The organism constantly integrates information about its internal state with external environmental stimuli. Adaptive brain and body interactions ensure optimal physiological homeostasis, which critically determines lifespan.
Research Area 3 concentrates on studying the mechanisms regulating the interaction between organisms and the environment and how these affect health and disease, in particular, aging and aging-associated pathologies.
Key neuronal networks in the hypothalamus provide a crucial integration point where neurons are specialized to sense hormones and nutrients as well as to respond to environmental stimuli and coordinate a broad spectrum of physiological responses. Impairments of these responses and processes can lead to the onset and development of major ageing-associated diseases.
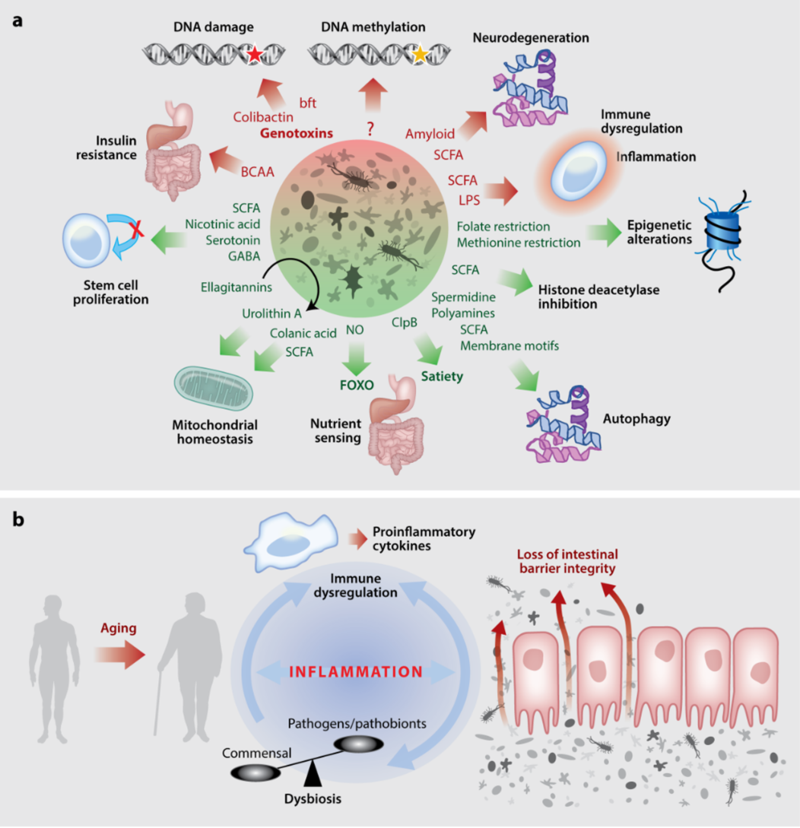
These include the findings of the Bergami group that glial cells have a crucial function in enabling vascular remodeling following acute brain injury (Gobel et al., Cell Metab. 2020) and discovery of a novel role for self-recycling process in the brain, important for neurodegeneration by the Kononenko group (Negrete-Hurtado et al., Nat Commun. 2019). Further, RA-3 scientists developed a novel method to study temporal and local dynamics of dopaminergic transmission, crucial for reward signalling, in humans (Lippert et al., Nat Commun. 2019). A collaboration of the Cornely, Bruning and Tittgemeyer groups characterized pathways enabling immediate orosensory and delayed post-ingestive reward system activation to promote food intake in humans (Thanarajah et al., Cell Metab. 2019). Further, recent studies have linked metabolic and neurological conditions with the composition of microbiota, which represents a critical interface between the organism and its environment. The diversity of the gut microbiota is linked with host immune, metabolic, as well as neurological conditions. The content and diversity of microbiota alters during aging and in response to dietary changes as well as some medical treatments. Work from the Cabreiro lab shows that microbes through their regulatory action on drug pharmacokinetics impacts host metabolism and ageing (Pryor et al., Cell 2019) and behaviour (Klunneman et al., Nature, 2021). Deregulation of the host-microbiota crosstalk during aging results in an elevated inflammatory state often referred to as ‘inflammaging’, which is implicated in the pathogenesis of aging-associated diseases. The Pasparakis group shed light on the mechanisms by which impaired barrier function causes microbiota-driven cytokine responses and intestinal inflammation (Eftychi et al., Immunity. 2019, Schwarzer et al., Immunity. 2020). In addition, scientists of the Kronke, Pasparakis and Kashkar groups discovered novel mechanisms regulating cell death and inflammation. Caspase-8 was identified as a molecular switch controlling apoptosis, necroptosis and pyroptosis (Fritsch et al., Nature. 2019), whereas macrophage necroptosis was shown to trigger inflammasome-dependent arthritis (Polykratis et al., Nat Cell Biol. 2019). Moreover, sensing of endogenous Z-nucleic acids by ZBP1 was identified as a key mechanism driving cell death and chronic inflammatory responses in epithelial tissues (Jiao et al., Nature. 2020).


Prof. Dr. Filipe Gomes Cabreiro
Principal Investigator
Head of Research Area 3

Prof. Dr. Tatiana Korotkova
Principal Investigator
Institute Director
Head of Research Area 3