
Prof. Dr. Carien Niessen
Scientific Coordinator, Principal Investigator,
Department Cell Biology of the Skin
carien.niessen[at]uni-koeln.de
Dermatologische Klinik, Zentrum für Molekulare Medizin Köln (ZMMK)
CECAD Research Center
Joseph-Stelzmann-Str. 26
50931 Cologne
The Niessen laboratory asks how control of cell shape and adhesive interactions coordinate formation and maintenance of the skin barrier that protects us from water loss and external insults, such as UV damage by the sun or microbes. Alterations in tissue architecture underlie aging-associated diseases, such as cancer and impaired regeneration. The team has unraveled central mechanisms by which stem cells balance renewal and differentiation to maintain the skin barrier. A major future goal is to gain fundamental insights into how regulation of cell architecture coordinates inflammatory and metabolic responses to control tissue homeostasis.
Our research: How epithelia coordinate cell and tissue structure with growth and metabolic activity to control morphogenesis and homeostasis of tissues is a fundamental question in biology. Altered regulation of epithelial cell/tissue architecture disturbs tissue homeostasis and epithelial barrier function and promotes not only aging but also cancer and a range of inflammatory diseases. The Niessen laboratory asks how key regulators of the cyto-architecture control the formation and maintenance of epithelial tissues. More specifically, the team addresses how cadherin mediated-cell-cell adhesion and aPKC-controlled polarity integrate with metabolic insulin/IGF-1 signaling to control stem cell renewal and differentiation, tissue integrity, and barrier function in a stratifying epithelium, the epidermis of skin. The Niessen laboratory also asks how external insults such as UV radiation present in the sun alter these processes and whether this results in skin barrier defects and diseases such as cancer.
Our successes: The scientific team around Prof. Niessen has provided important contributions to identify external and internal cues that control stem cell fate decisions in the epidermis likely through regulation of oriented cell division that promote either renewal or differentiation. These results not only provide cues for how stem cell behavior is altered in aged or diseased skin, but might open up novel therapeutic possibilities to rejuvenate stem cells in these conditions in the long term. Moreover, the Niessen laboratory has made significant advances in the molecular circuitry through which cadherins control the polarization and integrity of the epidermal barrier. This provides new insight into how interference with adhesion or polarity complex building blocks result in cancer and in autoimmune, genetic, and inflammatory diseases in the skin and other epithelial tissues.
Our goals: The long-term goal of Prof. Niessen and her team is to gain a fundamental understanding of the molecular mechanisms that drive polarization in multi-layered epithelia to coordinate cell fate, growth, and innate immunity with cell and tissue architecture. This will be central for understanding how stem cells balance self-renewal with differentiation to maintain proper barrier function during normal tissue turnover, and how this balance is restored upon insults/damage to the tissue to prevent disease.
Our methods/techniques: The research group of Prof. Niessen combines mouse models with 2D and 3D cell culture models to explore mechanistic insights into control of cell and tissue structure. With the help of state-of-the-art (life) imaging, these models allow the scientists to address the impact of interfering with the cyto-architecture of cells and tissues on the formation, maintenance and restoration of the epidermal barrier, and follow the individual fate of cells. We combine this with phospho-proteomics and genome-wide analyses to identify downstream mechanisms that in the long run may potentially serve as therapeutic targets.

Prof. Dr. Carien Niessen
Scientific Coordinator, Principal Investigator,
Department Cell Biology of the Skin
carien.niessen[at]uni-koeln.de
Dermatologische Klinik, Zentrum für Molekulare Medizin Köln (ZMMK)
CECAD Research Center
Joseph-Stelzmann-Str. 26
50931 Cologne
We have open PHD/Post-Doc positions!
If you are enthusiastic about skin research and interested in joining our laboratory, please contact Prof. Carien Niessen or Dr. Stéphanie Miceli.
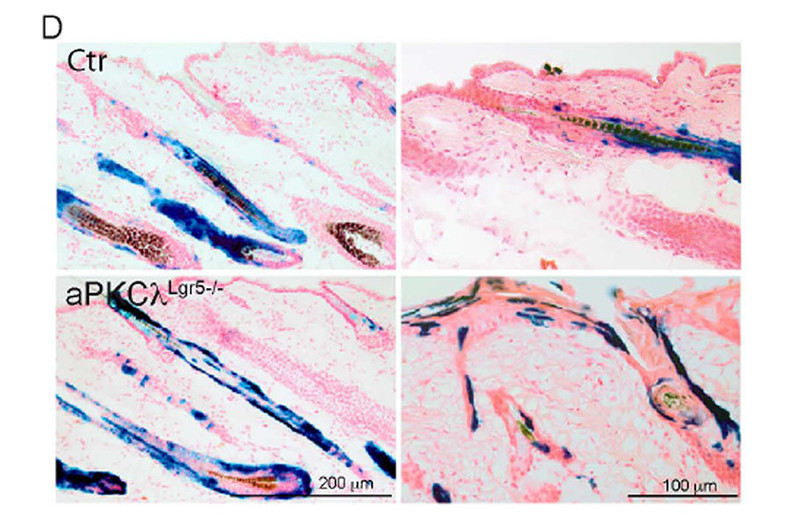
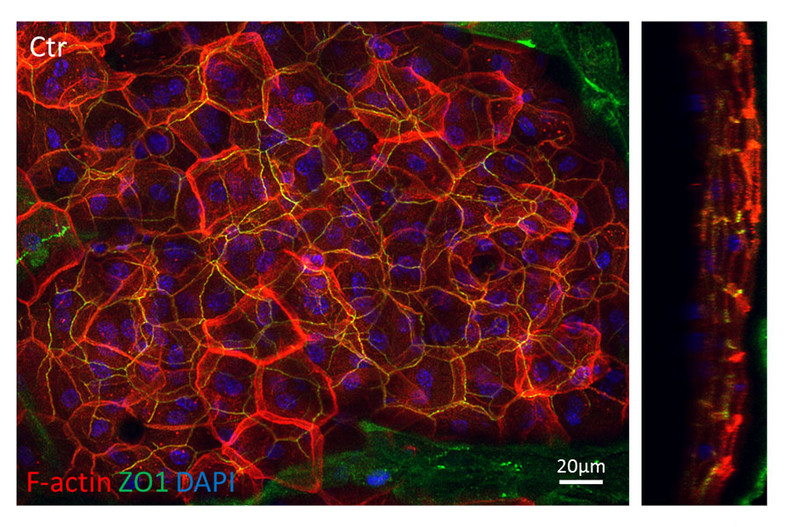
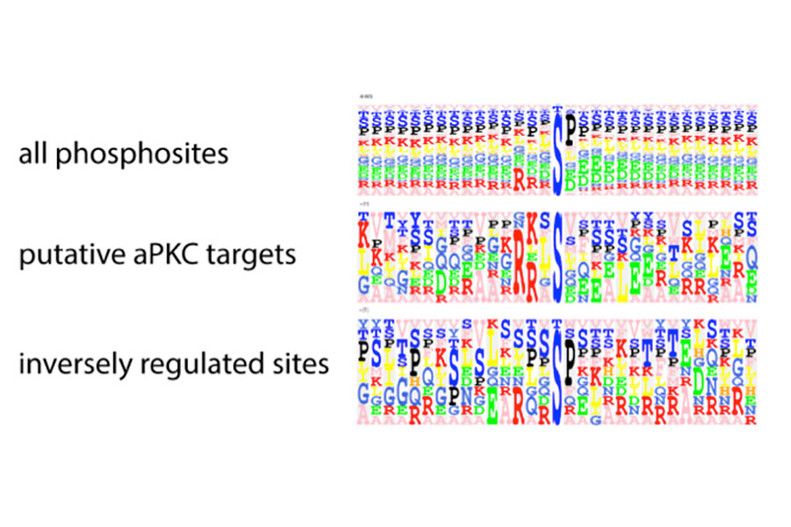
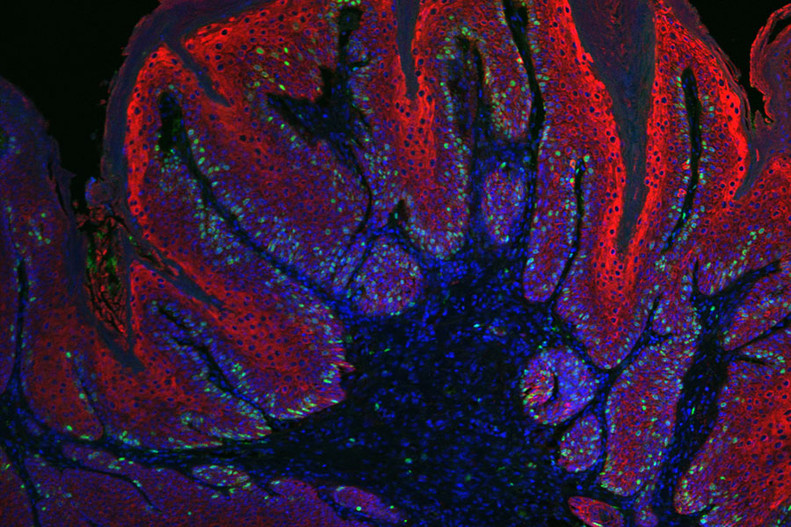
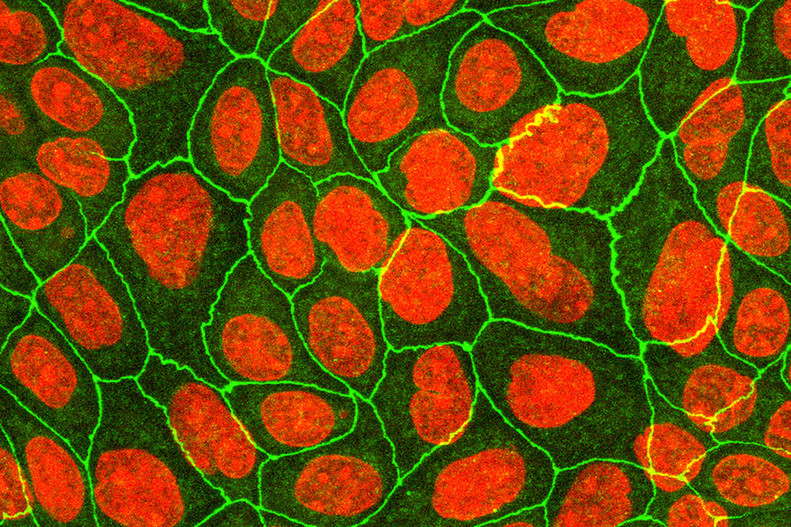
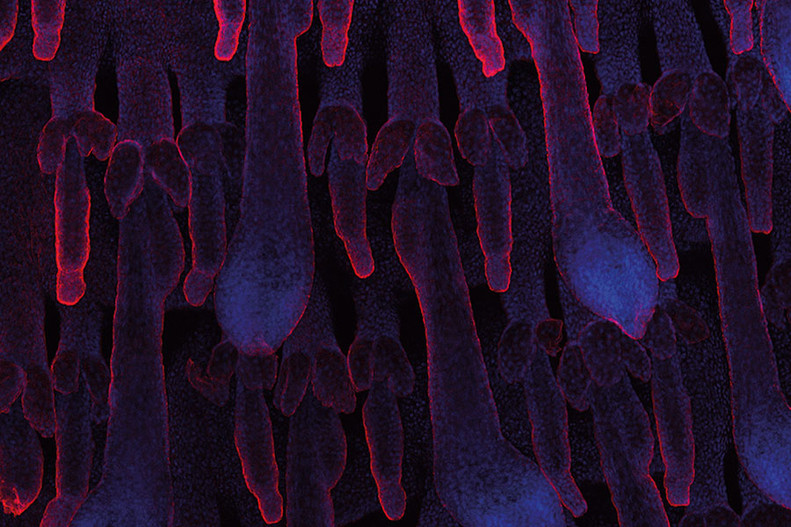
Figure 1: Lineage tracing of descendents of bulge stem cells (labeled in blue) showing that upon loss of aPKC stem cells change fate and obtain upper hair follicle and interfollicular identity as indicated by the blue staining in these areas.
Figure 2: Whole mount of newborn skin showing that F-actin (red) shows a polarized distribution across the different layers of the epidermis with the highest concentration above the tight junctional barrier labeled by ZO-1 (green). Right panel: XZ- projection.
Figure 3: Phosphorylation sites identified through SILAC/mass spectrometry analysis that are reduced upon loss of aPKC are enriched for aPKC consensus sites, indicating that these are putative aPKC targets.
Figure 4: Skin papilloma stained for K14 (red), BrdU (green) and nuclei (blue) showing enhanced proliferation in these tumors.
FIGure 5: Differentiated keratinocytes stained for ZO-1 (green) and nuclei (red).
Figure 6: Whole mount of adult tail skin stained for K14 (red) and nuclei (blue) visualizing hair follicles morphology.